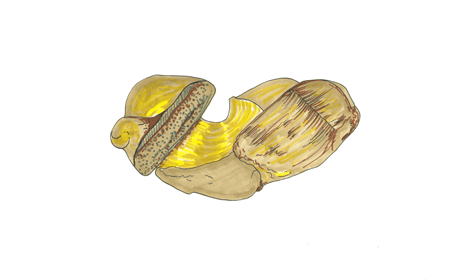
Mineral: Palladium
Chemical Formula: Pd
Critical Element: Palladium (Pd).
|
Mineral: Platinum
Chemical Formula: Pt
Critical Element: Platinum (Pt).
|
Discovery: Palladium was discovered by William Hyde Wollaston in 1803.
Main Uses: Catalytic converters, electronics, jewellery, dentistry and catalysts in chemical production.
Top Producers: Russia, South Africa, Canada, US and Zimbabwe.
Geological Setting: Palladium is produced as a by-product of nickel and copper production from some ores. It can also occur in large igneous bodies, where the dense metal accumulates at the bottom of a magma chamber.
Future Demand: Many Western governments have been discouraging the use of diesel cars. This has lead to an increase in the manufacture of petrol cars, which use palladium in the catalytic converter, thus increasing demand. While this demand may decrease again as electric cars become more popular, supply is not expected to increase which may lead to short term supply issues.
Environmental Issues: Mining palladium uses large quantities of water, a lot of energy and produces waste rock. Palladium extraction as a by-product is better for the environment than mining specifically for the metal.
|
Discovery: Platinum has been used in South America for centuries to make artefacts and ornaments.
Main Uses: Catalytic converters, jewellery, chemicals and oil refinery electronics.
Top Producers: South Africa, Russia, Zimbabwe, Canada and US.
Geological Setting: Economic platinum and palladium are produced as by-products of nickel and copper alongside palladium. The majority comes from large igneous bodies, where the dense metal accumulates at the bottom of the magma chamber.
Future Demand: While demand for platinum in cars is reducing, this is projected to be outweighed by the demand for platinum in jewellery. Supply is decreasing slightly as mines close in South Africa, which may lead to an increase in the cost of platinum.
Environmental Issues: Platinum mines in South Africa can have negative social and environmental impacts as people are displaced from the land that is mined on.
|
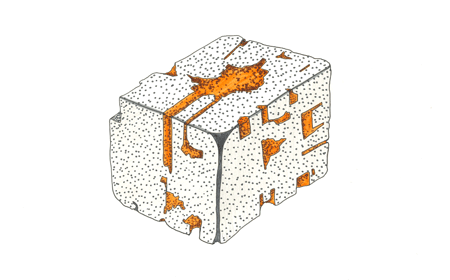
Mineral: Pyrochlore
Chemical Formula: (Na,Ca)2Nb2O6(OH,F)
Critical Element: Niobium (Nb).
|
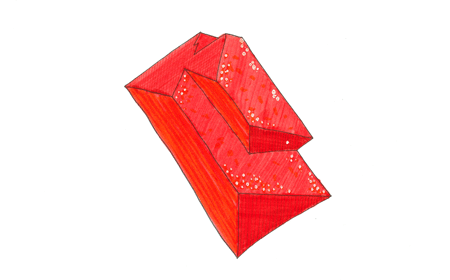
Mineral: Pyrolusite
Chemical Formula: MnO2
Critical Element: Manganese (Mn) 
|
Discovery: Pyrochlore was first described in 1826 in Norway.
Main Uses: Niobium is used to strength steel, jet and rocket engines, MRI scanners and camera lenses.
Top Producers: Brazil and Canada.
Geological Setting: Niobium is found in igneous rocks and in pegmatites. Pegmatites are coarse-grained igneous rocks that typically exhibit extreme variations in grain-size, from a few millimetres up to many metres. Pegmatites are typically considered to be residual melts that form during the late-stages of crystallisation of a body of magma. As such they represent the final portion of a magma to crystallise and tend to be enriched in incompatible elements, for example lithium, tantalum, niobium, and caesium. Incompatible elements are those that are not readily incorporated in major rock-forming minerals such as quartz and feldspar, because of their unsuitable ionic radii (size) and/or charge.
Future Demand: Projections suggest that current niobium reserves should meet demand for the next 500 years.
Environmental Issues: Thorium is a radioactive by-product of pyrochlore mining and is damaging to the environment.
|
Discovery: Pyrolusite was named in 1827 from the Greek for ‘fire’ and ‘to wash’ because it removed tints in the making of glass.
Main Uses: Steel manufacture, alloyed with aluminium and added to unleaded gasoline
Top Producers: South Africa, Australia, China, Gabon and Brazil.
Geological Setting: Pyrolusite is concentrated in hydrothermal veins, where manganese bearing minerals are oxidised by the warm, circulating water. Pyrolusite can also form in bogs.
Future Demand: Manganese is being used in the batteries of electric cars, increasing demand. However, changes in the way China produces its steel have meant that manganese is used less. Stocks are enough to keep the price of manganese stable in the near future.
Environmental Issues: Manganese is toxic in high concentrations. Studies have shown that children living near manganese mines have detrimental health effects as a result of mining.
|
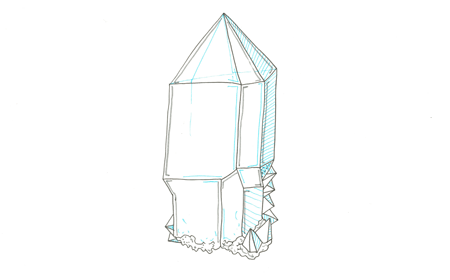
Mineral: Quartz
Chemical Formula: SiO2
Critical Element: Silicon (Si)
|
Mineral: Rutile
Chemical Formula: TiO2
Critical Element: Titanium (Ti)
|
Discovery: The first recorded use of the word ‘quartz’ was in the 14th Century.
Main Uses: Computers, phones, microchips and building materials.
Top Producers: China, Russia, USA, Norway and France.
Geological Setting: Quartz is the second most common mineral on the Earth’s crust after feldspar and can be found in almost any rock types. Quartz is the dominant component of sand and is therefore most easily seen on a beach!
Future Demand: Quartz is so common that supply will always exceed demand.
Environmental Issues: Most silicon is mined in open cast pits from quartz mineral veins. This quartz is used industrially without being purified, often with little processing from its natural form.
|
Discovery: Named in 1800 by Martin Heinrich Klaproth.
Main Uses: Alloyed into steel, alloyed with aluminium, refractive lenses, medicine and dentistry.
Top Producers: China, Russia, Japan, Kazakhstan and Ukraine.
Geological Setting: Rutile can be found in igneous rocks that have been altered by metamorphism and in pegmatites. Pegmatites are coarse-grained igneous rocks that typically exhibit extreme variations in grain-size, from a few millimetres up to many metres. Pegmatites are typically considered to be residual melts that form during the late-stages of crystallisation of a body of magma. As such they represent the final portion of a magma to crystallise and tend to be enriched in incompatible elements, for example lithium, tantalum, niobium, and caesium. Incompatible elements are those that are not readily incorporated in major rock-forming minerals such as quartz and feldspar, because of their unsuitable ionic radii (size) and/or charge.
Future Demand: Titanium is an abundant metal, with relatively stable demand.
Environmental Issues: The process of refining titanium often involves chlorine which must be safely disposed of to avoid environmental damage.
|
Mineral: Sphalerite
Chemical Formula: (Zn,Fe)S
Critical Elements: Zinc (Zn), Indium (In) and Germanium (Ge)
|
Mineral: Spodumene
Chemical Formula: LiAl(SiO₃)₂
Critical Element: Lithium (Li)
|
Discovery: Named in 1800 by Abraham Gottlob Werner.
Main Uses: Sphalerite is the main ore used to obtain zinc but it also contains trace amounts of rarer metals, such as indium and germanium.
Zinc is used to galvanise metals, it’s used in alloys with brass, bronze and aluminium, and it’s also used to make coins. Indium is used in TVs, solar power cells and in phone screens. Germanium is also used in solar power cells. It is added to glass for lenses used in cameras, microscopes and thermal imaging cameras.
Top Producers: China, Peru, Australia, US and Canada.
Geological Setting: Sphalerite is a relatively common mineral and can be found in igneous, sedimentary and metamorphic rocks. The most common economic deposits are either:
- Hydrothermal, where sphalerite is dissolved in warm, saline water before being deposited as temperatures drop.
- Contact metamorphism, where an igneous intrusion is so hot that it cooks the surrounding rock that it is intruding into.
Future Demand: While demand for zinc has remained relatively consistent since 2013, known deposits of sphalerite will keep up with demand for a long time.
Environmental Issues: As with all sulphide minerals, mining sphalerite can cause acid mine drainage. However, zinc recycling is becoming more common, rising from 6% to 25% of zinc consumption over the last fifteen years.
|
Discovery: First described in 1800 by Brazilian naturalist, Jose Bonifacio de Andrada e Silva.
Main Uses: Electric and hybrid vehicles, batteries including many personal electronics, ceramics, lubricating greases, and air treatment
Top Producers: Australia, US, and China.
Geological Setting: Spodumene normally forms in lithium-rich granite pegmatites. Pegmatites are coarse-grained igneous rocks that typically exhibit extreme variations in grain-size, from a few millimetres up to many metres. Pegmatites are typically considered to be residual melts that form during the late-stages of crystallisation of a body of magma. As such they represent the final portion of a magma to crystallise and tend to be enriched in incompatible elements, for example lithium, tantalum, niobium, and caesium. Incompatible elements are those that are not readily incorporated in major rock-forming minerals such as quartz and feldspar, because of their unsuitable ionic radii (size) and/or charge.
Future Demand: Total has predicted that electric vehicles will account for 30% of the market by 2030. Making the batteries for all those electric cars would require a 6-fold increase in lithium supply. While enough identified deposits do exist, they may not be able to bring production online in time to meet this accelerating demand.
Environmental Issues: Lithium batteries come with a fairly high environment cost. However, they may play an important role in reducing our dependency on fossil fuels and may also have good recycling potential.
|
Mineral: Stibnite
Chemical Formula: Sb2S3
Critical Element: Antimony (Sb)
|
Mineral: Tantalite
Chemical Formula: (Fe,Mn)Ta2O6
Critical Element: Tantalum (Ta)

|
Discovery: Stibnite has been used by humans since the Ancient Egyptians, who used it as a medicine and cosmetic.
Main Uses: Flame retardant, batteries, alloyed with lead and tin to improve hardness and infrared detectors.
Top Producers: China, Russia, Tajikistan, Bolivia and Australia.
Geological Setting: Stibnite mainly occurs in hydrothermal deposits, where warm water collects stibnite from large volumes of rocks and deposits them where the water cools, thus concentrating the mineral into an ore body. It can also be found in volcanic rocks and sandstones.
Future Demand: Antimony consumption has remained relatively stable in recent times. Reserves are good for the metal. If this were to change, antimony can also be substituted relatively easily in it’s uses as a flame retardant and alloy.
Environmental Issues: The mining of antimony does not have any notable effects on the environment. Although antimony is a toxic metal, it’s solubility causes it to become widely distributed, dissipating its negative effects.
|
Discovery: The metal tantalum was discovered in Sweden in 1802 by Anders Ekeberg.
Main Uses: Phones, DVD players, games consoles and computers.
Top Producers: Democratic Republic of Congo, Brazil, China, Rwanda and Mozambique.
Geological Setting: Tantalum mainly occurs in granite pegmatites and their placer deposits. Pegmatites are coarse-grained igneous rocks that typically exhibit extreme variations in grain-size, from a few millimetres up to many metres. Pegmatites are typically considered to be residual melts that form during the late-stages of crystallisation of a body of magma. As such they represent the final portion of a magma to crystallise and tend to be enriched in incompatible elements, for example lithium, tantalum, niobium, and caesium. Incompatible elements are those that are not readily incorporated in major rock-forming minerals such as quartz and feldspar, because of their unsuitable ionic radii (size) and/or charge.
Future Demand: Changes in the demand for products that tantalum is used in has caused a strange trend in use, falling dramatically in 2011 and rising slowly since. Known reserves are projected to meet future demand.
Environmental Issues: Tantalum is a conflict mineral in the Democratic Republic Congo. In 2003, a United Nations report linked tantalum production in the Coltan province to the funding of militant groups, however this province provides a very small percentage of tantalum globally.
|
Mineral: Wolframite
Chemical Formula: (Fe,Mn)WO4
Critical Element: Tungsten (W)

|
|
Discovery: Tungsten was first named in 1546 by Georg Agricola in his book, ‘De Re Metallica’. He named the mineral ‘Lupi Spuma’, or ‘wolf’s froth’ in English. Wolframite was named by Johan Gottschalk Wallerius, a Swedish scientist, in 1747.
Main Uses: Rocket engines, phones, X-ray tubes, radiation shielding and alloys.
Top Producers: China, Russia, Canada, Bolivia and Vietnam.
Geological Setting: Wolframite can occur in pegmatites. Pegmatites are coarse-grained igneous rocks that typically exhibit extreme variations in grain-size, from a few millimetres up to many metres. Pegmatites are typically considered to be residual melts that form during the late-stages of crystallisation of a body of magma. As such they represent the final portion of a magma to crystallise and tend to be enriched in incompatible elements, for example lithium, tantalum, niobium, and caesium. Incompatible elements are those that are not readily incorporated in major rock-forming minerals such as quartz and feldspar, because of their unsuitable ionic radii (size) and/or charge.
Future Demand: China produced 55% of global tungsten and therefore has a high control on the price of the metal. Many countries do not publish information on their consumption, reserves or recycling of tungsten to maintain their negotiating power when trading.
Environmental Issues: Tungsten has very low environmental toxicity but was classified as a conflict mineral in the Democratic Republic of Congo.
|
|