China and Russia currently monopolise the global supply of scandium. Pete Siegfried, Frances Wall and Kathryn Moore examine the distributions of scandium globally and identify a number of promising targets that could help diversity supply.
Siegfried, P., Wall, F. & Moore, K., In search of the forgotten rare earth.
Geoscientist 28 (10), 10-15, 2018
Scandium (Sc) has been called a ‘miracle metal’. When alloyed with aluminium, it produces super-strong but lightweight materials, just right for use in the next generation of aeroplane manufacture and other high-tech applications. By definition, Sc is a member of the 17-strong, rare earth element (REE) family. Like the other REEs, Sc isn’t actually rare in terms of distribution—it can be found throughout Earth’s crust and has a similar abundance to lead—but unlike lead, economic concentrations of Sc are very rare, making it one of the most expensive elements in the world. Indeed, concentrations of Sc are usually so low that Sc is often excluded from geological assessments of REE—it is the forgotten rare earth.
But this may be about to change. Several research groups are turning their attention to Sc, using new data from exploration projects to identify a number of promising sources that could be mined. Here we discuss the behaviour of Sc and the distribution of these deposits worldwide. We argue that with improved understanding of how Sc associates with clinopyroxene and how it concentrates within weathering and waste products, such as laterites and red muds, these newly identified deposits could create a virtuous circle of raw materials supply and new high-tech uses.
Why Scandium?
The manufacturing industry requires a wider range of specialist raw materials than ever before, especially for digital and green technologies. In this context, Sc is used in solid oxide fuel cells, lasers, ceramics, neutron screens, lighting, high-specification sports equipment and amelioration of the glare from spotlights.
Current interest in Sc is driven mainly by projected use in the aerospace industry. Al-Sc alloys are strong, light and weldable. They have been used for years by NASA and the Federal Space Agency, where price was no problem, so have been tried and tested. Only tiny amounts of Sc are needed for alloys, which then create lighter, more fuel-efficient aircraft and cars. These are not only cheaper to run, but emit less CO
2, helping nations achieve long-term emission targets set by policy makers. If a ready supply were available, Sc could revolutionise the aerospace and automotive industries.
Although potentially revolutionary in terms of its applications, global demand for Sc is currently small, at around 10-15 tonnes annually, according to the United States Geological Survey. Sc exists in such low concentrations that it is difficult and expensive to extract, hampering use in commercial applications. But, this is something of a chicken-and-egg scenario. There is no shortage of applications for Sc, so if reasonably cheap and assured supplies of Sc become available, more applications will be found and demand will increase.
Sc is mainly produced as a limited by-product of conventional uranium and nickel extraction processes. The main producers of Sc are China, Russia, Ukraine, and the Philippines (Fig. 1), and production in Russia and China is thought to be accelerating. In particular, there are ongoing plans to considerably increase Sc production from waste dumps at the Bayan Obo mine in China, which will serve to strengthen China’s monopoly of the whole rare earth market.
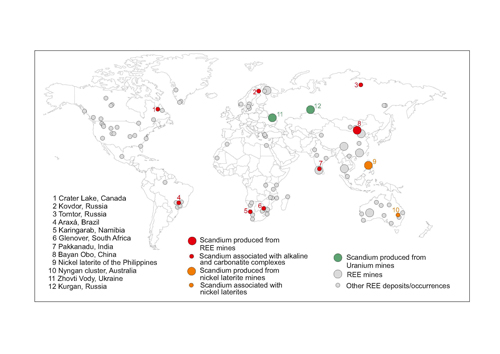 Fig. 1, Sc mines and deposits compared with REE mines and deposits (courtesy of the British Geological Survey)
Fig. 1, Sc mines and deposits compared with REE mines and deposits (courtesy of the British Geological Survey)
In contrast, there are currently no known, economically viable, large-scale Sc resources in the USA or western Europe, and many companies are dependent upon Sc single-sourced either from Russia or China. Such lack of diversity in supply could lead to economic issues if the current supplies are jeopardized—an increasingly likely scenario, given current political tensions surrounding Brexit, the US and Europe, and China, leading to a fear of future global trade wars. A trade dispute relating to the export of REEs occurred in 2010, for example. Low labour costs and lax environmental restrictions meant China could more easily and cheaply produce REE than other nations, and they became the world’s dominant supplier. In 2010, the Chinese government reduced its export quotas by 40%, arguing that this was needed to protect the environment. REE prices outside of China soared. China finally dropped the export quotas in 2015 following a ruling from the World Trade Organisation that the restrictions violated trade regulations. But the dispute was a wake-up call, a reminder of the need for secured supply of essential elements, particularly in Europe. And several projects are currently underway. One is the SCALE EU project (
http://scale-project.eu/scandium), which aims specifically to develop a stable and secure European supply chain for Sc using bauxite and titania acid wastes that can serve European aerospace and high-tech industries.
Sc properties
Sc is a soft, silvery transition metal. It is officially defined as part of the REE family together with yttrium (Y) and the lanthanoids (also called lanthanides). However, the smaller size of the Sc3+ cation causes rather different geochemical behaviour to the rest of its family members, so many general rules about the geochemistry of REE do not apply to Sc (Fig. 2). Most reviews of REE geochemistry (e.g. Chakhmouradian and Wall, Elements 2012), mention this fact and then exclude Sc from further consideration. The United States Geological Survey treats Sc separately from the REEs. So, Sc has been rather forgotten in the REE literature.
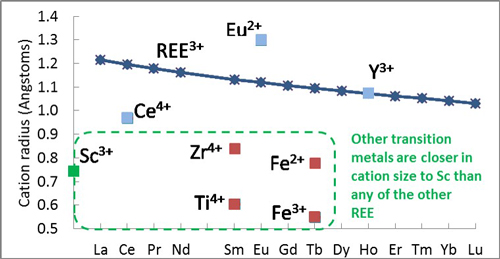 Fig. 2, Plot of REE atomic number vs. cation radius. Sc3+ is not part of the ‘lanthanoid contraction’ (REE3+ cations decrease in size as atomic number increases), it is much smaller than the other REEs and closer in size to transition metals, such as Ti, Fe and Zr (included here in arbitrary positions on the x axis). Cation radii from http://abulafia.mt.ic.ac.uk/shannon/radius.php?
Fig. 2, Plot of REE atomic number vs. cation radius. Sc3+ is not part of the ‘lanthanoid contraction’ (REE3+ cations decrease in size as atomic number increases), it is much smaller than the other REEs and closer in size to transition metals, such as Ti, Fe and Zr (included here in arbitrary positions on the x axis). Cation radii from http://abulafia.mt.ic.ac.uk/shannon/radius.php?
Sc rarely concentrates in any geological setting and the rocks with highest concentrations of Sc are of extra-terrestrial in origin! Lunar basalts and chondritic meteorites are enriched by a factor of three compared with terrestrial rocks. On Earth, the highest concentrations of Sc are chiefly associated with occurrences of high-field-strength elements, such as titanium, tantalum and zirconium, especially in granite pegmatites. Indeed, granite pegmatites provide a small amount of the Sc produced commercially each year. An ore deposit of Sc may well contain concentrations of just a few hundred parts per million (ppm) Sc in the rock. This is higher than a gold deposit, which may contain only one ppm gold, but much lower than copper or the other main REE, such as neodymium (Nd).
Bayan Obo in China—the world’ largest REE mine—is a source of Sc (Williams-Jones and Vasyukova,
Economic Geology 2018). This seems at odds with our assertion that Sc does not follow the other REE in natural environments until we learn that the Sc comes from pyroxene re-processed from the waste tips at Bayan Obo, rather than from the REE ore minerals themselves. Indeed, figure 1 shows that highly anomalous Sc chemistry also appears to be associated with some alkaline silicate rocks and with carbonatites, but REE deposits are not necessarily Sc deposits.
Sc in minerals
Of the 5,000-odd species in the mineral kingdom, only sixteen are Sc minerals, and these are all rare. The most common Sc mineral, thortveitite, occurs in granite pegmatites (Table 1). It contains the highest weight percent Sc at 32% and has been mined as a Sc ore in the past.
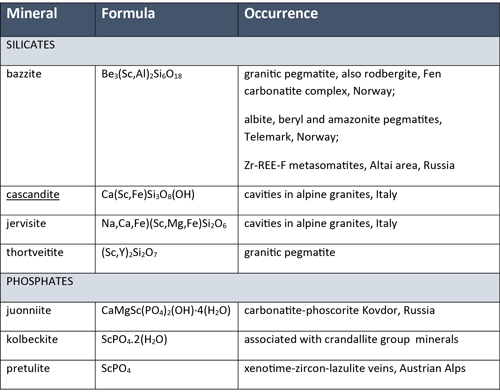 Table 1, Examples of scandium minerals
Table 1, Examples of scandium minerals
Sc also occurs as a minor component in hundreds of minerals, substituting for titanium, zirconium, iron or tantalum. Other important Sc-bearing minerals likely have names unfamiliar to most geologists and include scandium-columbite, davidite, tantalite, samarskite, ixiolite, rutile, Ti-aeschynite, zircon, catapleiite and baddeleyite. Additionally, Sc isn’t always incorporated into a mineral structure, and may instead be adsorbed onto the surfaces of iron oxide and hydroxide minerals.
Sc in laterites
Laterites are iron- and aluminium-rich rocks and soils formed by a prolonged process of chemical weathering. At least four Sc laterite deposits in eastern Australia are undergoing feasibility studies that may bring them into production as mines. The Sc was hosted originally in clinopyroxene and then released and adsorbed onto iron oxides during the strong weathering process that produced the laterite. The Nyngan laterite deposit in New South Wales, Australia, developed by weathering of basic rocks. It has grades up to 409 ppm Sc and has been touted as the world’s first stand-alone Sc deposit.
The mineralogy of the weathering process has been studied in the Syerston–Flemington deposit of New South Wales. This Co+Ni+Sc deposit contains about 1,350 tonnes of Sc at an average concentration of 434 ppm. Mathieu Chassé recently found that deposit formation requires a high initial concentration of Sc in clinopyroxene. These are then weathered leading to Sc-rich waters circulating below the water table. Seasonal precipitation allows the adsorption of Sc3+ onto goethite, which accounts for about 80 % of the Sc budget (Fig. 3). The deposit contains up to 800 ppm Sc in its limonitic laterite compared to about 80 ppm in its parent rock. This exceptional concentration of Sc in lateritic deposits requires a combination of three circumstances: (1) anomalously high Sc concentration in the parent rock, (2) long time-scales of alteration in a stable tectonic environment and (3) lateritic conditions during weathering, allowing the trapping of Sc by Fe oxides.
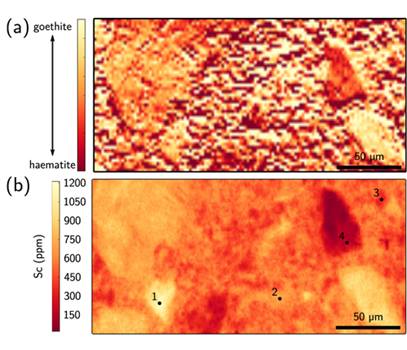 Fig. 3, Maps of the oxidation state of iron, interpreted as goethite and hematite (a) and distribution of Sc (b). Maps made by Mathieu Chassé et al. (2016) using synchrotron µ-XRF and used to propose that Sc is mainly hosted by goethite in the Syerston–Flemington Ni-Co laterite deposit. (Credit: Chassé et al, 2016, Geochemical Perspectives Letters 3, 105-114; under Creative Commons Attribution 4.0 License)
Fig. 3, Maps of the oxidation state of iron, interpreted as goethite and hematite (a) and distribution of Sc (b). Maps made by Mathieu Chassé et al. (2016) using synchrotron µ-XRF and used to propose that Sc is mainly hosted by goethite in the Syerston–Flemington Ni-Co laterite deposit. (Credit: Chassé et al, 2016, Geochemical Perspectives Letters 3, 105-114; under Creative Commons Attribution 4.0 License)
Sc in red muds
‘Red muds’ are the waste products of aluminium processing after bauxite ores have been treated by the Bayer process. Vast tailings dams of this material occur in many parts of the world and there has been recent interest in recovering REE from this waste product. This is one of the few environments where REE, including Sc, are concentrated together. Grades of about 100 ppm Sc have been found in red muds in Greece waste and so research is ongoing (e.g
http://scale-project.eu/) to determine if any economic recovery processes for Sc can be found.
Sc in carbonatites and alkaline rocks
Carbonatites (igneous carbonate rocks) and alkaline rocks (some of the most bizarre and extreme composition igneous silicate rocks) are the main types of deposits mined currently for REE, so we turned to existing geochemical databases to see if these rocks typically contain high concentrations of Sc. According to the GeoRoc database (
http://georoc.mpch-mainz.gwdg.de/georoc) a single carbonatite sample from Pakkanadu, India revealed a Sc content of 237 ppm. A sample from Bayan Obo, China had a high Sc value of 111 ppm.
The REE exploration boom of 2010 to 2013, and the increase of the traded price of Sc from $2,000 to $5,000/kg in 2012, encouraged a number of mining companies to include Sc in their initial resource calculations. Our review of these results, however, supports the idea that REE-rich carbonatites and alkaline rocks do not necessarily contain high concentrations of Sc (although some localities are worthy of future attention).
The best-known example of Sc enrichment in carbonatite is probably the carbonatite-phoscorite deposit at Kovdor, Kola Peninsula, Russia (Fig. 4). This deposit is mined for magnetite, apatite and baddeleyite (ZrO2). Sc substitutes for Zr in baddeleyite (Kalashnikov and colleagues, Ore Geology Reviews 2016), which contains an average of 780 ppm Sc.
 Fig. 4, The phoscorite at Kovdor mine, Kola Peninsula, Russia has spectacular relationships between apatite, magnetite, olivine and calcite-rich rocks but Sc is contained in baddeleyite, a mineral rarely seen in the field.
Fig. 4, The phoscorite at Kovdor mine, Kola Peninsula, Russia has spectacular relationships between apatite, magnetite, olivine and calcite-rich rocks but Sc is contained in baddeleyite, a mineral rarely seen in the field.
Africa’s best Sc potential may be in the Glenover pyroxenite-carbonatite complex in South Africa, with a Sc content of 300-500 ppm in a supergene apatite-martite breccia (Fig. 5). The complex was mined for apatite, while the low-grade ore was stockpiled rather than processed. This stockpiled material is of interest for its REE—including Sc—potential. The REE are hosted mainly in monazite but the Sc is hosted in zircono-silicates, aeschynite and secondary Nb-oxides. Sc-bearing aeschynite is now also recognised in the carbonatite and regarded, together with aegirine, as an important host for the primary Sc mineralisation.
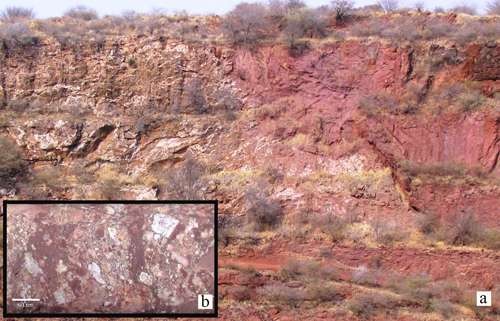
Fig. 5, The Glenover open pit mine, South Africa. (a) Secondary red coloured apatite-martite breccia (RHS) formed through weathering of carbonatite veins intruding apatite pyroxenite (LHS). Height of profile is approximately 20 m. (b) Diamond drill core sample of apatite-martite breccia containing angular white and cream coloured secondary apatite clasts.
Recent exploration work by Imperial Mining on the Crater Lake alkaline complex located in Quebec, Canada, outlined significant Sc hosted in a magnetic ferro-syenite. The main host to this Sc is the clinopyroxene, hedenbergite.
Values of nearly 450 ppm Sc were reported in the weathered Karingarab carbonatite in Namibia (Fig. 6), suggesting that weathering is a key factor in concentrating Sc. Tomtor (Yakutia, Russia) is a huge deposit of weathered carbonatite with exciting grades of niobium and REE, including average grades of 391 ppm Sc. Weathered carbonatites at Araxá and Catalão (Brazil), Mt Weld (Australia), Sukulu (Uganda), Mabounié (Gabon) and Sokli (Finland) all may be of interest for their Sc potential.
 Fig. 6, Examining drill core at Karingarab, Namibia. This material may contain as much as 437 ppm Sc.
Fig. 6, Examining drill core at Karingarab, Namibia. This material may contain as much as 437 ppm Sc.
The future for Sc
Although classed as a REE, the smaller cation size of Sc means that it doesn’t behave in the same way and its mineral hosts are not the same as for the other REE. So, we can’t use current understanding of REE behaviour to identify promising deposits of Sc and shouldn’t assume that REE-rich deposits will contain high concentrations of Sc. Our assessments of the concentrations of Sc in various rock types and deposits globally show that Sc associates with niobates, titanates, iron oxi/hydroxides and, importantly, clinopyroxene. Focused study of Sc content in clinopyroxene is therefore a necessary avenue for further research, and it may be worth looking again at previously investigated alkaline complexes and carbonatites. Successful, cheap and easy extractive metallurgy needs to be developed and fine-tuned to target this important potential host.
Potential Sc deposits exist in Canada, Namibia, Brazil, Australia, Uganda, Gabon, and in Europe, Finland. Clearly, there are a number of promising deposits of Sc that could increase and diversify supply, and potentially reduce overreliance on China and Russia.
Given the numerous applications of this ‘miracle metal’, Sc exploitation seems ripe for development. Research time and funds should be devoted to identifying viable Sc deposits in more western nations to secure supply, as well as to better understand how Sc can be more efficiently extracted from its many different sources. There is still plenty for geoscientists to do to improve our understanding of the geology, geochemistry and mineralogy of this rather neglected element. If successful, we could be on the cusp of a Sc boom.
Note: All three authors are researchers on the EU H2020-funded HiTech AlkCarb project, grant number 689909. Pete Siegfried is also an independent consultant and has carried out consulting at the Glenover, Karingarab, Araxá and Catalão localities mentioned in this article.
Pete Siegfried
1, Frances Wall
2 and Kathryn Moore
2
1. GeoAfrica, P.O. Box 24218, Windhoek, Namibia
2. Camborne School of Mines, University of Exeter, Penryn Campus, Cornwall, TR10 9FE
Further reading:
Chakhmouradian, A.R. & Wall, F. (2012) Rare earth elements: minerals, mines, magnets (and more),
Elements (Ottawa): an international magazine of mineralogy, geochemistry, and petrology 8, 333-340.
Chassé, M., Griffin, W.L., O’Reilly, S.Y. & Calas, G. (2016) Scandium Speciation in a World-Class Lateritic Deposit.
Geochemical Perspectives Letters 3, 105-114.
Kalashnikov, A.O., et al. (2016) Scandium of the Kovdor baddeleyite–apatite–magnetite deposit (Murmansk Region, Russia): Mineralogy, spatial distribution, and potential resource.
Ore Geology Reviews 72, 532–537.
U.S. Geological Survey (2017) Mineral Commodity Summaries, January 2017, p146-147,
https://minerals.usgs.gov/minerals/pubs/commodity/scandium/mcs-2017-scand.pdf (accessed January 2018).
Van der Walt, G. N & Dabrowski, F. A. (2012) Geological Report and Resource estimate for the Glenover carbonatite project. Geoconsult International, 140 pp.
http://www.galileoresources.com/reports.htm (accessed June 2018).
Williams-Jones, A. E. & Vasyukova, O. V. (2018) The Economic Geology of Scandium, the Runt of the Rare Earth Element Litter.
Economic Geology 113, 973-988.