 Linda Campbell, Mark Tyrer and Alan Dyer* explore some emerging new insights into the geological and planetary occurrences of this common element.
Linda Campbell, Mark Tyrer and Alan Dyer* explore some emerging new insights into the geological and planetary occurrences of this common element.
Any attempt to inspire geologists with enthusiasm for a common and almost ubiquitous element in the Earth’s crust has to be slightly audacious! Nevertheless, as world population is predicted to hit nine billion by 2050, and as demand for fertilizers from mined K-resources increases1 there is an imperative to better understand natural K-cycling. While potash resources, evaporites and authigenic mineral reactions have largely (and appropriately) been the domain of sedimentologists, it is now time for the wider mysteries of K concentration and cycling to be revisited across a wider range of geological disciplines.
Picture - some K-rich foodstuffs. Spinach, bananas and dried apricots are good, too...
MYSTERIES
 Within the last decade, an esteemed sedimentologist allegedly commented “…but we still don’t know where the potassium comes from”, in relation to brine sources and authigenic K-feldspar replacement, in a cross-continent study. This is an intriguing and compelling comment about a common element which follows the fundamental rules of chemical behaviour in geology, and which is so familiar to us all in the chief rock-forming minerals. The comment is mirrored by news of the abundant occurrence of ultramafic-associated jarosite, KFe3(SO4)2(OH)6, on Mars.
Within the last decade, an esteemed sedimentologist allegedly commented “…but we still don’t know where the potassium comes from”, in relation to brine sources and authigenic K-feldspar replacement, in a cross-continent study. This is an intriguing and compelling comment about a common element which follows the fundamental rules of chemical behaviour in geology, and which is so familiar to us all in the chief rock-forming minerals. The comment is mirrored by news of the abundant occurrence of ultramafic-associated jarosite, KFe3(SO4)2(OH)6, on Mars.
Picture - Mars Rover 'Opportunity' discovered the K-mineral jarosite on the Red Planet in 2004. (Courtesy NASA/JPL-Caltech).
Jarosite (pictured below, right) usually forms in acidic environments from the microbially-mediated oxidation of pyrite, but it is now heading for spotlight attention, not least because of its Martian occurrence and relationship to water, but also because of reports of an uncharacteristic occurrence in a high-pH saline lake environment on Earth2. It is thought that the saline lake occurrence is also the result of pyrite breakdown, where acidic conditions prevail just at the mineral surfaces. Continental lake evaporites, supplying K resources mainly from the mineral sylvite, have compositions distinctively different from seawater-derived evaporites. They are discussed in a detailed review3, in which some, but not all of the mysteries of potash deposits are explained in relation to patterns of ages and tectonic settings.
 These seemingly diverse topics all drive research questions towards the concentration and cycling of K in magma-mineral-fluid systems. It is here, and especially in East African studies, that we find a focus of expertise on rift-associated alkaline environments4,5,6, and where perhaps some clearer understanding might be found.
These seemingly diverse topics all drive research questions towards the concentration and cycling of K in magma-mineral-fluid systems. It is here, and especially in East African studies, that we find a focus of expertise on rift-associated alkaline environments4,5,6, and where perhaps some clearer understanding might be found.
CROSS-DISCIPLINARY
A new hypothesis and conceptual model has been introduced7 on the shared links between alkaline rocks, zeolite minerals and carbonatites (igneous rocks with >50% carbonate minerals – see John Barry Dawson's Obituary, this issue). Presented as a potential resource pathfinder innovation (especially for rare earth elements (REE) from carbonatite suites), and noting the implications for estimating volcanic contributions of carbon to the atmosphere (see https://dco.gl.ciw.edu/news), the globally applicable hypothesis outlines a natural ‘masquerade’ scenario. Essentially, it is proposed that the continental, bedded zeolites represent ‘hidden’ alkaline pyroclastic deposits, most probably originally accompanied by magmatic carbonate.
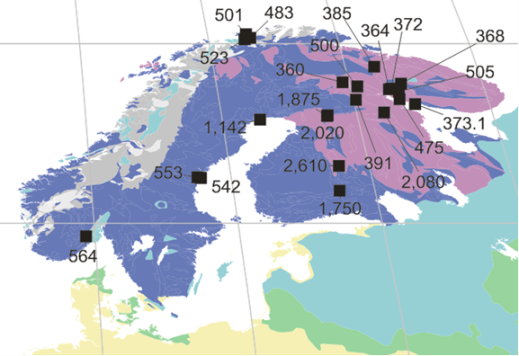
Map: Scandinavia map from Woolley & Kjarsgaard5 showing reptition of carbonatite occurrence with time. Ages in Ma.
 If the model is examined in the context of the hot spring saline lakes of East Africa, the brines can be shown to be related to the fluid plumbing system of the magmatism (K-rich in places) in this region. So, to better understand evaporitic potash, we can look to investigations of the mineral reactions occurring in these dynamic surface environments, where contact with the atmosphere changes lava crusts within hours of eruption8. The interplay between Na and K during these processes helps to explain how K is concentrated in evaporite deposits, as chlorides (e.g. sylvite and carnallite) and in some K-rich zeolites such as phillipsite, clinoptilolite and merlinoite, derived from bedded tuffs.
If the model is examined in the context of the hot spring saline lakes of East Africa, the brines can be shown to be related to the fluid plumbing system of the magmatism (K-rich in places) in this region. So, to better understand evaporitic potash, we can look to investigations of the mineral reactions occurring in these dynamic surface environments, where contact with the atmosphere changes lava crusts within hours of eruption8. The interplay between Na and K during these processes helps to explain how K is concentrated in evaporite deposits, as chlorides (e.g. sylvite and carnallite) and in some K-rich zeolites such as phillipsite, clinoptilolite and merlinoite, derived from bedded tuffs.
PRESERVATION
 Picture: Ol Doinyo Lengai hornitos of natrocarbonatite lava, East Africa Rift, studied by Anatoly Zaitsev8.
Picture: Ol Doinyo Lengai hornitos of natrocarbonatite lava, East Africa Rift, studied by Anatoly Zaitsev8.
Nowhere else is the preservation issue of these silica-undersaturated igneous rocks so strikingly demonstrated as in the present day East African volcanics. For carbonatite science however, the preservation-pattern of extrusive products in the geological record is problematic because carbonate might have other, non-magmatic sources, and the alkali elements Li, Na, K, Rb and Cs are highly mobile. Add to this, the huge diversity of alkaline rock types plus the subsequent domination of Si-buffered alteration fluids in continental settings, and it is plain to see that the waters become muddied (literally – with clay minerals!). Perhaps the masquerade hypothesis7 could prove to be helpful, highlighting the contribution of natural zeolites as transient ‘staging posts’ en route to more stable, preserved assemblages (e.g. with K-feldspar) in longer geological histories?
Natural processes therefore demonstrate that alkaline alteration is critical to the staged release of K from an aluminosilicate environment to a hydrated one, where it readily becomes exchangeable in a diverse suite of zeolite minerals. The known value of zeolites in agricultural applications also becomes evident9, and it comes as no surprise that alkaline rocks are being trialled as fertilizers due to their high reactive susceptibility, releasing their K relatively easily from feldspathoid minerals1. For micas, K is readily released by ion-exchange.
VALUE OF ZEOLITE
 Picture: Moab potash evaporation ponds, near the Colorado River, Utah. Image: Google Earth.
Picture: Moab potash evaporation ponds, near the Colorado River, Utah. Image: Google Earth.
Generally, the major element compositions of zeolites provide a clue as to the compositions of the precursor components. Classic examples are the K-rich zeolites montesommaite, merlinoite, phillipsite, chabazite and erionite from the ultrapotassic region of central Italy, and Ba-rich edingtonite in zeolite assemblages from carbonatite localities in the Kola peninsula, Russia, Mont St. Hilaire and Ice River in Canada, and Jacupiranga in Brazil. Zeolite formation is favoured by high pH, low-Si conditions, and for the abundant, economic, continental-bedded types, alkaline volcanic glass compositions are ideal precursor materials4. Where zeolites have not been subject to progressive reaction processes, their trace element compositions also appear to preserve alkaline and peralkaline REE signatures too7.
Using the revelatory evidence from the carbonatite community of spatially-consistent repetition of alkaline-carbonatite magmatism over vast periods of geological time (billions of years5), a superb opportunity for resource exploration beyond evaporitic potash deposits exists. Young zeolites associated with continental extensional tectonic regimes, especially where continental saline lake environments are identified, are suggested as obvious prime target regions for mineral exploration, because ancient systems that have concentrated the ores could have occurred in the same places. The Mountain Pass REE deposit, lying under a large province of young bedded zeolites is a case in point, but in isolation, is merely interesting rather than serving as convincing evidence. With global applicability, the zeolitic masquerade model therefore provides considerable scope for locality-specific testing where known deposits occur, including deposits of non-marine potash.
FUTURE
 Picture: Clinoptilolite, a K-rich zeolite.
Picture: Clinoptilolite, a K-rich zeolite.
The scientific value of K in understanding Earth system science is considerable. So to what extent can we work backwards along zeolitic and evaporite-mineral reaction paths to reach alkaline-carbonatitic rock compositions in bedded successions that are difficult to explain with conventional models? The abundance and widespread occurrence of zeolite minerals (particularly K-rich varieties and their potential contributions to geological problem-solving and to resource needs) therefore demand renewed attention. More background data are needed to create 21st Century impacts from the zeolitic pathfinder hypothesis, and these are achievable with wider-academic and industrial engagement.
Recent rejuvenation of interest in NE England marine potash resources10 is undoubtedly symptomatic of the global revitalization of fertilizer demand. With fundamental scientific research potential and the driving force of increasing global resource needs, getting excited about potassium is timely.
Acknowledgements
Anatoly Zaitsev for Ol Doinyo Lengai hornitos - with permission license from Elsevier. Scandinavian carbonatites map - open access, Geological Survey of Canada. Other figures from Wikipedia Commons and Linda Campbell.
References
- Manning, D A C (2012) Plant Nutrients. In: Hester R E and Harrison, R M (Eds.) Soils and Food Security. Issues in Environmental Science and Technology, 35, 183-197.2.
- McHenry, L J, Chevrier, V and Schröder, C (2011) Jarosite in a Pleistocene East African saline-alkaline paleolacustrine deposit: Implications for Mars aqueous geochemistry, J. Geophys. Res., 116, E04002, 15pp.
- Warren, J K (2010) Evaporites through time: Tectonic, climatic and eustatic controls in marine and nonmarine deposits. Earth-Science Reviews 98, 217–268.
- Bish D, Ming D (eds) (2001) Natural Zeolites: Occurrence, Properties, Applications. Rev. Mineral. Geochem. 45. Mineralogical Society of America and Geochemical Society, Washington DC.
- Woolley, A R Kjarsgaard B A (2008a) Carbonatite Occurrences of the world: map and database. Geol Surv Can, Open File 5796. 1 CDROM + 1 map.
- Woolley A R, Kjarsgaard, B A (2008b) Paragenetic types of carbonatite as indicated by the diversity and relative abundances of associated silicate rocks: evidence from a global database. Can Mineral 46, 741-752.
- Campbell, L S, Dyer, A, Williams, C and Lythgoe, P R (2012) The masquerade of alkaline carbonatitic tuffs by zeolites: A new global pathfinder hypothesis. Mineralium Deposita, 47, 371-382.
- Zaitsev A N and Keller J (2006) Mineralogical and chemical transformation of Oldoinyo Lengai natrocarbonatites, Tanzania. Lithos 91, 191–207.
- Ramesh, K., Reddy, D D, Biswas, A K et al. (2011) Zeolites and their potential uses in agriculture. Advances in Agronomy, 113, 215-236.
- www.bbc.co.uk/news/uk-england-york-north-yorkshire-21484936 See also, www.pratley.com/ for K-rich clinoptilolite.
Authors
 Dr Linda Campbell is an independent researcher at The University of Manchester ([email protected]).
Dr Linda Campbell is an independent researcher at The University of Manchester ([email protected]).
 Dr Mark Tyrer is an independent geochemist, based in Derbyshire and London. He is a Research Manager for MIRO, the Mineral Industry Research Organisation, Visiting Professor of geomaterials at Coventry University and Honorary Research Fellow at Imperial College. [email protected].
Dr Mark Tyrer is an independent geochemist, based in Derbyshire and London. He is a Research Manager for MIRO, the Mineral Industry Research Organisation, Visiting Professor of geomaterials at Coventry University and Honorary Research Fellow at Imperial College. [email protected].

Professor Alan Dyer is a Scientific Consultant based in Lancashire. He is a past Research Professor at the University of Salford and Visiting Professor at Loughborough University.
[email protected].