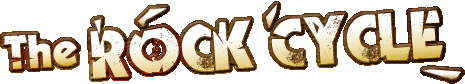Sediments produced by Evaporation
 There are many chemical salts dissolved in sea water, originally formed by weathering of rocks. The most important are Calcium carbonate (CaCO3), calcium sulphate (CaSO4), and sodium chloride (NaCl).
There are many chemical salts dissolved in sea water, originally formed by weathering of rocks. The most important are Calcium carbonate (CaCO3), calcium sulphate (CaSO4), and sodium chloride (NaCl).Oolitic limestone
Oolitic limestone is a formed by carbonate deposition in very shallow, warm waters like those around the Bahamas today (see satellite photo).In these shallow waters, waves gently roll the grains on the sea floor, so they grow in layers like an onion. The Cotswold hills are made of oolitic limestone

Gypsum
Gypsum (calcium sulphate) is a soft white mineral which often forms by evaporation along low-lying shores like those of the Arabian Gulf. It is the main ingredient of plaster and plaster-board.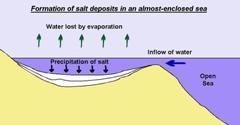
Rock Salt
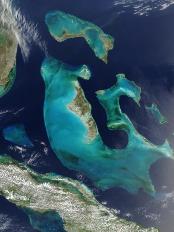
Rock salt (sodium chloride) has many uses in the chemical and food industries, and a lot goes on our roads in winter to prevent ice from forming. Some is formed in playa lakes (see page on Hot Deserts), but the largest deposits form where an almost-enclosed area of shallow sea loses water by evaporation in hot, dry conditions. The sea doesn’t dry out because more water flows in… and lots of salt is deposited.