 Diveena Danabalan and colleagues* ponder the origin, transport and accumulation of that most elusive element.
Diveena Danabalan and colleagues* ponder the origin, transport and accumulation of that most elusive element.
Helium is the second most abundant element in the universe, and yet here on Earth it is a more limited commodity. Supplies come from only four countries. The USA has dominated the market for decades but in the last few years it has been joined by Algeria, Qatar and Russia. Superficially, this would appear to be a good development, but supply of helium to the western world is precarious.
Picture above, below: 210 years ago this October, the Geological Society was founded. And so, 10 years ago, in January 2007, the Society opened its Bicentennial celebrations by the release of 4567 biodegradable natural latex balloons at Burlington House, by permission of the Civil Aviation Authority. But should we have worried more about the waste of precious helium?
Why is this a problem? Helium is not only used in party balloons and advertising blimps. Cryogenics accounts for around 32% of global demand, with medical cryogenics (such as MRI scanners) becoming ever more important and abundant. For these there is no adequate substitute for He – it really is an elemental hero. The Large Hadron Collider, for example, requires 120 tonnes of superfluid helium to maintain its magnets at their operating temperature of 1.9K. A new truckload is required to arrive in Geneva each week, to top it up. Helium is also extensively used in arc welding, purging, leak detection, chromatography, super-conduction, breathing mixtures, heat transfer, gas lift; and (extensively) as a purge gas in the oxygen/hydrogen propulsion units used for space rocketry and nuclear ICBMs. All current He reserves were discovered by accident. What is needed is a strategy for finding this precious commodity on purpose.
Reserves in the USA have declined significantly in recent years, while those in Qatar, although massive, are entirely dependent upon the Liquefied Natural Gas (LNG) market remaining robust, because helium is a by-product of the cryogenic process needed to produce it. This, combined with the strategic risk of relying on a small number of geopolitically sensitive suppliers, makes western markets nervous. Indeed, typing ‘helium’ into a search engine produces a stream of articles about ‘helium shortage’.
 Recycling is becoming more common, but containing helium is difficult and leakage from storage inevitable – it is the ultimate ‘Houdini’ element. Once 'free', not even gravity impedes it. It even escapes the Earth's atmosphere and is lost to space – the very reason it is so rare on Earth.
Recycling is becoming more common, but containing helium is difficult and leakage from storage inevitable – it is the ultimate ‘Houdini’ element. Once 'free', not even gravity impedes it. It even escapes the Earth's atmosphere and is lost to space – the very reason it is so rare on Earth.
Current global helium, the result of serendipitous discoveries, is a by-catch from the search for petroleum. Indeed, it was one such chance discovery that first alerted John Gluyas to the problem. Back in 1999 John, an oil company geologist, changed employer; and the first new task awaiting him was to evaluate the plan for a gas field development in southern Asia. The plan was fine but he was intrigued by a single line in a table, labelled ‘gas composition’ listing He as a constituent gas. A quick bit of maths revealed that once the field was on production it would deliver c. 0.25 million standard feet per day of helium. This was globally significant. (Alas, ahead of any substantive plan to exploit it, an even bigger company took over, and it would seem nothing happened. The field is now on stream producing methane, nitrogen and a small proportion of helium.)
A few years before, in 1996, Chris Ballentine was working in the University of Michigan and awarded a grant by the US Department of Energy to study the geochemistry of helium-bearing natural gases in the Hugoton-Panhandle natural gas field. There was little sense then that helium was running out, as demand had not yet accelerated to today’s level – but the study (published in 2002) resulted in the most comprehensive understanding of commercial helium occurrence at that time.
That you might be able to find helium on purpose remained a pipe-deream for a decade, before Gluyas and Ballentine first discussed a possible helium exploration strategy. In December 2011 helium users in Europe and the US received letters from suppliers; helium was in short supply. This concentrated minds, and, early in 2012, the two pitched their idea to Statoil of Norway. Shortly after, a research award to develop a helium exploration strategy was awarded to the universities of Durham and Manchester, and I was subsequently appointed to undertake it. Statoil veteran Tony Doré quipped that it must have been the first time ever that Statoil had invested in anything with no calorific value!
Origins
 Any exploration strategy must depend on an understanding of origins. Helium was first discovered in economic quantities in Dexter, Kansas, USA in 1903 where it was referred to as the ‘gas which would not burn’. Analysis of the gas from this field found that it was dominated by nitrogen but also contained 1.84% helium by volume. Such an amount is uncommon; in most fields helium occurs in traces (0.05% or lower).
Any exploration strategy must depend on an understanding of origins. Helium was first discovered in economic quantities in Dexter, Kansas, USA in 1903 where it was referred to as the ‘gas which would not burn’. Analysis of the gas from this field found that it was dominated by nitrogen but also contained 1.84% helium by volume. Such an amount is uncommon; in most fields helium occurs in traces (0.05% or lower).
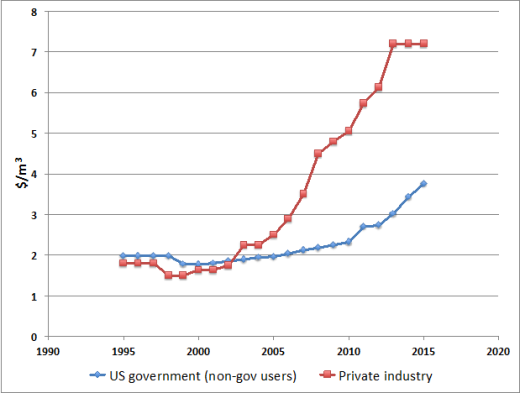
Picture: Helium consumption in the US by sector
The discovery allowed its properties to be explored, which in turn led to its widespread use in physics and chemistry. Markets developed. Other discoveries were made, in Wyoming, Oklahoma, Texas, Kansas, New Mexico, Utah, Arizona, Montana and Colorado. Some fields contained up to 10% He by volume – significant, when the economic concentration for extractable He is 0.3% - at which level its value is about the same as the remaining 99.7% of the discovered gas volume (assuming this is saleable methane).
Other discoveries of helium-rich gas have also been made (in Canada, Algeria, Poland, Russia, Germany, Hungary, Romania, Qatar, Kazakhstan, India, Pakistan, China and the Timor Sea). However none of these new reserves currently matches the concentrations or volumes found in the USA. To date only 14 fields across 12 countries actively produce and contribute to the world’s supply of the gas.
So, current reserves are set to decline to critically low levels in a few tens of years. We have been failing to replace what we use. Recycling is minimal and largely unfeasible. Moreover, we suspect that many gas production companies are unwittingly venting He because they either do not test their gas or, if they do, fail to recognise its value. We have evidence that helium is indeed being vented from ‘off-spec gas’, rich in non-combustible compounds, during the clean-up process.
As a result, while global demand has steadily risen (proportional to a price increase of c. 175% in the last decade for Grade A helium), crude helium is now worth approximately 30 times the equivalent volume of crude methane, and has been priced as between $3.75/m3 (raw He) and $7.21/m3 (Grade A He, refined to a purity of c. 99.997% or better).
Where is helium found?
 The two stable isotopes of He differ by only one neutron. On Earth, 4He is the most common and demands the majority of industrial interest. 3He, the much rarer isotope (3He/4Heair = 1.4x10-6) has limited specialist applications, not least in neutron detectors in airports, and a candidate for power generation though nuclear fusion power. 3He is often referred to as ‘primordial helium’ due to the bulk of it being trapped in the mantle during the Earth’s formation, with few mechanisms to produce it via radioactive-linked processes.
The two stable isotopes of He differ by only one neutron. On Earth, 4He is the most common and demands the majority of industrial interest. 3He, the much rarer isotope (3He/4Heair = 1.4x10-6) has limited specialist applications, not least in neutron detectors in airports, and a candidate for power generation though nuclear fusion power. 3He is often referred to as ‘primordial helium’ due to the bulk of it being trapped in the mantle during the Earth’s formation, with few mechanisms to produce it via radioactive-linked processes.
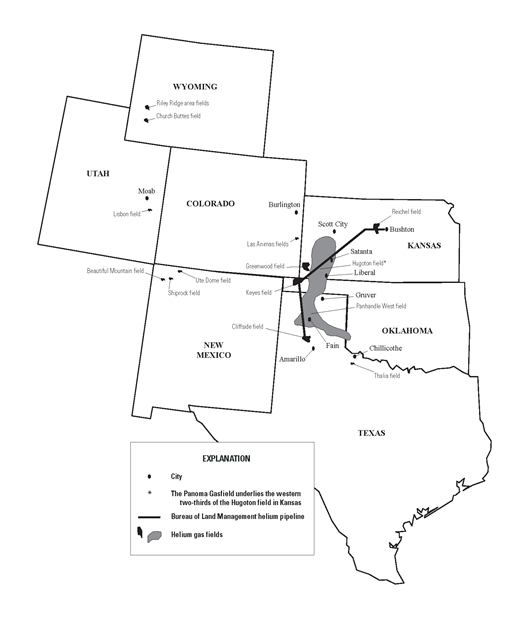
Picture: Map showing helium-rich gas fields and He processing plants in the United States, 2012. From USGS.
3He/4He in the Earth’s mantle is enriched in 3He relative to air (3He/4Hemantle = 1.12x10-6), and these higher isotopic ‘mantle’ ratios can be readily measured in hydrothermal systems at mid ocean ridges and many volcanic gases. However it remains at concentrations far too low to be naturally sourced, with commercial quantities limited to the (small) by-product of nuclear power plants.
Conversely, the more abundant isotope, 4He, is produced by alpha decay of U and Th in the crust (the alpha particles stabilise to become 4He), which is why it is also called ‘radiogenic helium’. In this sense it is perfectly ‘renewable’, albeit only on a geologic timescale. Different rock types produce varying amounts of 4He, depending on original concentrations of U and Th and age of the rock - with some of the highest production found in Precambrian crystalline terranes such as the Canadian Shield. So although helium’s escapology puts quicksilver to shame, it is constantly being created.
Helium is found in groundwater, natural gas fields, ancient brines, fluid inclusions in ore deposits, hydrothermal fluids, igneous intrusions and rocks, oil field brines, lakes, ice sheets, oceanic sediments and coal measures. However, only a handful of natural gas fields contain enough He for extraction to be commercially viable.
Producing He-rich fields can be divided according to their primary gas component into three main types:
- N2-rich: - Harley Dome, Pinta Dome (USA)
- CO2-rich: – LaBarge, Doe Canyon (USA)
- CH4-rich: - North Dome-South Pars (Qatar/Iran), Hugoton-Panhandle (USA), Hassi R’Mel (Algeria)
The helium system
Perhaps the fact that all helium has been discovered by accident while searching for petroleum holds the solution to exploring for helium deliberately. Long ago oil and gas companies ceased trusting to chance and developed an exploration method whereby a basin or area is assessed by considering potential source rocks and regional reservoir/seal combinations. In short, we adapted well-established hydrocarbon exploration protocols to make helium the target, and so devised the following questions:
- How and where are helium generated (source rock)
- How is helium liberated from the source rock (primary migration)
- How does helium move significant distances from the source rock (secondary migration)
- How do pools of helium rich gas accumulate in the crust (trapping mechanisms)
- How are helium accumulations destroyed by nature (trap breaching and/or dissipation)
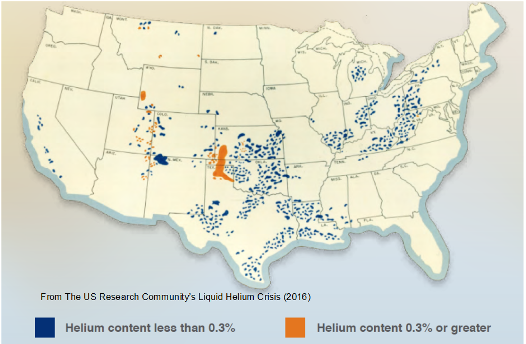 Picture: Helium content in US natural gas reserves
Picture: Helium content in US natural gas reserves
We have already mentioned the source of helium being rocks, especially granites, rich in thorium and uranium. We have mainly addressed primary migration, secondary migration and how helium accumulates. To do this we sampled existing helium producing areas in the US Mid-West and Canada. We attempted (unsuccessfully) to sample known He-rich gas wells in Italy and South Asia. Finally and most recently, by teaming up with exploration company Helium-One, we sampled He-rich seams in the Tanzanian section of the East African Rift. All samples were subject to rigorous gas composition analysis and of the isotopic composition of the separated He, other noble gases and nitrogen.
From a helium-source perspective, it is clear that older granites have had the time to produce more helium than younger ones. The first step in exploring for He is therefore to look for Archaean granitic/crystalline terrain. However, age is not the only determinant of a viable source; primary migration from that source needs to have occurred. High energy alpha particles carve 10-20 micron length ‘fission tracks’ in the uranium and thorium-bearing minerals, along which He atoms can migrate. While it is not yet clear how efficiently He escapes from the crystal lattice, it is probably quite efficient. Gas will then accumulate in fluid inclusions and fractures within the host rock.
For He to escape from the (low permeability) inclusions and fractures, however, requires energy – typically, heat. This could come from rifting, subduction, volcanic activity or similar processes; but small, dispersed amounts of He are still probably hard to mobilise. We don’t understand this primary release process very well; but is likely to be enhanced by the presence of a second carrier fluid or gas phase. Simply put, large amounts of fluid trapped in tight rock are easier to mobilise than small amounts, and any major gas or fluid phase would carry any helium with it.
Carrier
 We think we know what the carrier phase is. High helium concentrations in natural gases are always associated with high concentrations of nitrogen (N2/4He ratios are typically c. 10 to 50). Many natural gases that are nitrogen-rich contain little or no helium, though – which is simply explained by the fact that there are multiple sources of trapped nitrogen in sediments (e.g. petroleum source rocks and coal) from which N2 can be released by increases in temperature and pressure.
We think we know what the carrier phase is. High helium concentrations in natural gases are always associated with high concentrations of nitrogen (N2/4He ratios are typically c. 10 to 50). Many natural gases that are nitrogen-rich contain little or no helium, though – which is simply explained by the fact that there are multiple sources of trapped nitrogen in sediments (e.g. petroleum source rocks and coal) from which N2 can be released by increases in temperature and pressure.
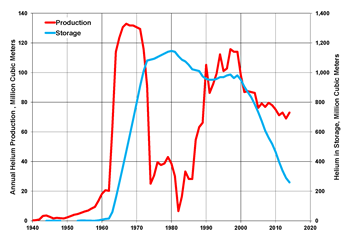
Picture: Helium production and storage in the United States, 1940-2014 (data from USGS)
Why radiogenic helium should be associated with non-radiogenic nitrogen is intriguing. It has been found that the isotopic composition of nitrogen associated with economic helium deposits is consistent with the range expected from low temperature metamorphism, and that crystalline rocks contain levels of nitrogen, often found as NH4+ in clays, that would support observed 4He/N2 ratios.
So, we think we have a basic idea of how helium and nitrogen are related, and how they escape from source rocks. What happens next?
Strong evidence from other noble gas isotopes (e.g. 20Ne and 36Ar, which only originate from air) that the admixture of helium and N2 is dissolved in groundwater in overlying lithologies. This is a problem. We need enough nitrogen and helium dissolved in the water so that, when it ascends, the system depressurises enough to form a separate nitrogen-helium gas phase – before reaching any trap structures. This may indeed have occurred where pure N2-He gas fields are found. However, it is also possible that the presence of significant concentrations of CO2 or CH4 in other helium-rich natural gas-trapping structures gives us an important clue to the nitrogen-helium migration story.
When groundwater that contains dissolved helium and N2 is equilibrated with existing CO2 or CH4 gases, the insoluble helium and N2 will exsolve from the groundwater into any pre-exsiting gas phase. In this case, a pre-existing gas phase composed of CO2 or CH4 is essential for ‘stripping’ groundwater containing accumulated but dissolved helium and N2 into any existing trapping structure. This doesn’t come without an added complication. Since CO2 and CH4 are some of the most prevalent gases in the subsurface, there is a risk is that where helium and N2 accumulate, the helium can be diluted by large amounts of associated gases to non-commercial concentrations.
If we are relying on CH4 and CO2 to strip dissolved helium/nitrogen, we need to establish the ‘goldilocks’ zone, where the degree of dilution is ‘just right’. For example: volcanic activity is a known source of high CO2 gas fields. Therefore, in the right geological setting, the thermal aureole associated with magmatism may provide the heating needed to release helium from its source – but if the trap is too close to the volcanic centre, all you are likely to find is CO2.
Once helium has migrated into a trap, the preservation of helium in that field depends on the rate at which helium is supplied to the deposit and the efficiency of the seal or trap to hold on to it.
What next?
Our database is growing but is still minute when compared with the accumulated knowledge for, say, petroleum exploration. We need to seek out and analyse more natural He occurrences to better understand migration and accumulation. We also still have to understand the systematics of helium accumulation destruction, and the temporal integrity of seals. More immediately (and certainly of more interest to many) will be our current work on mapping ‘helium play fairways’ around the globe. Where best might we explore for helium? Watch this space!
Further reading
- Ballentine, C.J. and Sherwood Lollar, B. (2002) Regional groundwater focusing of nitrogen and noble gases into the Hugoton-Panhandle giant gas field, USA, Geochimica et Cosmochimica Acta, 66, 2483–2497
- Cady, H.P. and McFarland, D.F. (1907) The occurrence of helium in natural gas and the composition of natural gas, J. Am. Chem. Soc., 29 (11), pp 1523–1536
- Danabalan, D., Gluyas, J.G., Macpherson, C.G., Abraham-James, T.H., Bluett, J.J., Barry, P.H. and Ballentine, C.J. (2016) New high-grade helium discoveries in Tanzania, Goldschmidt Conference Abstracts https://goldschmidtabstracts.info/abstracts/abstractView?id=2016004150
Authors
Diveena Danabalan1*, Jon Gluyas1, Colin Macpherson1, Pete Barry2 & Chris Ballentine2. 1: Department of Earth Sciences, Durham University, Elvet Hill, Durham, DH1 3LE, U.K., E: [email protected]. 2: Department of Earth Sciences, University of Oxford, South Parks Rd, Oxford, OX1 3AN, U.K.