Crystallisation of Magma
Crystallisation of magmas – Grain size & cooling rate using Salol (background)
In teaching science at KS3, students are usually happy to accept that coarse-grained igneous rocks result from slow cooling underground because “crystals have longer to grow”. Reality, alas, is more complex. When a liquid is cooled below its melting point, some degree of super-cooling will result. A clean test tube containing liquid salol (phenyl salicylate) may cool well below room temperature before crystallising at all. This is because the process of crystal nucleation is endothermic - it requires an energy input from its surroundings. However, crystal growth is exothermic - releasing energy to the surroundings, and thus retarding nucleation of new crystals by keeping the melt warm. In igneous intrusions, the insulating effect of the surrounding rock thus means that crystal nucleation may be very slow - because fewer crystals are able to nucleate, those that do are free to grow to larger size.
A good way to illustrate the release of heat by crystallisation of a melt is to use a “hand-warmer” (available from many Chemists’ shops), which contains a super-cooled melt; crystallisation is normally initiated by a mechanical “clicker”.
An experiment to illustrate the relationship between grain size and cooling rate is described in a separate worksheet, based on an experiment described on the JESEI and ESEU websites (see 'Useful Links' page).
In our experience this experiment works better if the salol is only just melted (m.p. 42oC). A water bath at 45oC should suffice. The experiment works best when a drop of molten Salol is sandwiched between two microscope slides. One pair should be cool (if too cold, then the salol crystallises before the sandwich is completed) and the other pair should be warm (can use a water bath - but wipe dry before use, or leave on a warm radiator). Crystal size can be viewed adequately using an OHP, but hand lenses or microscopes (low power) are better.
Salol cooled rapidly
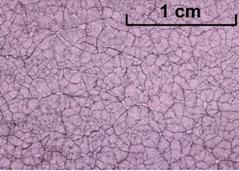 Salol cooled rapidly – small crystals (JESEI)
Salol cooled rapidly – small crystals (JESEI)Salol cooled slowly
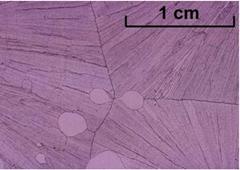 Salol cooled slowly – large crystals (JESEI)
Salol cooled slowly – large crystals (JESEI)